Beyond the basics: Understanding hemolysis
Preanalytical errors in blood gas testing have to be addressed holistically
What is Hemolysis?
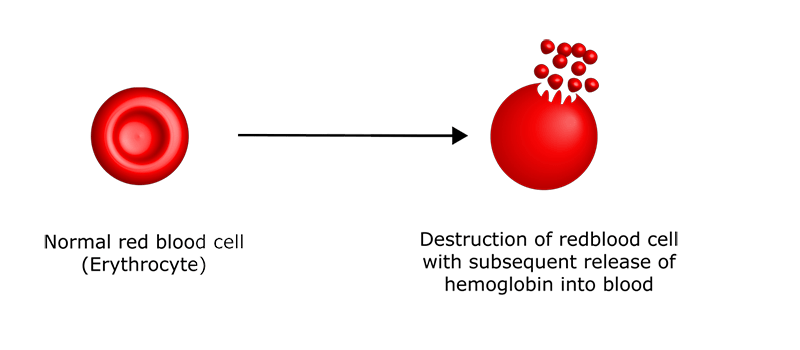
Hemolysis is the rupture of red blood cells, releasing their contents, including hemoglobin, into the surrounding fluid. This can occur naturally within the body due to pathological conditions (such as autoimmune hemolytic anemia or a transfusion reaction) (1) and is referred to as in vivo hemolysis.
On the other hand in vitro hemolysis is what occurs between blood sample collection to the sample measurement (e.g., too vigorous mixing of a sample, prolonged storage time, excess blood aspiration rate, inappropriate needle size, etc.) (2).

While detecting hemolysis is crucial to get alerted about the integrity of blood samples and the reliability of patient care decisions, it’s only one of the factors that impact overall sample quality. We recommend that preanalytical errors in blood gas testing are addressed holistically to always ensure the best possible quality of results and subsequent diagnosis and treatment decisions.
Preanalytical errors in blood gas testing have to be addressed holistically to always ensure the best possible quality of the results and subsequent diagnosis and treatment decisions
Preanalytical errors account for between 60% and 97%* of all errors observed (3, 4)
The preanalytical phase can be broken down into several steps, e.g.:
- ✔ Choice of appropriate blood gas sampling device
- ✔ Sample labeling and patient data transcription
- ✔ Sample collection
- ✔ Sample handling, and
- ✔ Preparation for analysis
* Not blood gas specific but including blood gas samples
While there are many preanalytical errors encountered in the above steps, some of the most common are clotting, hemolysis, insufficient mixing, and patient sample mix-up (5). These can lead to several negative outcomes from bias on various parameters (e.g., insufficient mixing leads to bias on total hemoglobin results) to blockage of the sample pathway in the analyzer (in the case of a clotted sample).
We recommend healthcare professionals to focus on adopting best practices to minimize preanalytical errors. Standardized procedures, proper training, and adherence to best practices remain the most effective strategies for reducing errors and ensuring accurate test results.
Beyond one error: understanding testing challenges
While preanalytical errors as a whole contribute to a significant proportion of total testing errors, studies have shown that the frequency of hemolysis in blood gas samples can vary (4, 6, 7). For instance, a recent study from 2022 found hemolysis in 3.5% of ICU blood gas samples (8).
Overall hemolyzed sample found in the ICU
Studies have shown that some of the errors can be mitigated via selection of the right devices, and implementation of education and training programs

Preanalytical excellence
The bigger picture: beyond hemolysis
While hemolysis detection is critical for identifying samples with impaired integrity, it’s just one of many potential preanalytical errors that can compromise results. Errors such as patient mix-up, inadequate sample mixing, room air contamination, and risk of clots are equally important to address. If not addressed, preanalytical errors can lead to numerous issues, including the need for sample recollection, unnecessary additional investigations, dissatisfaction with healthcare services, increased costs, misdiagnoses, prolonged turnaround times (TAT), delayed diagnoses or treatments, and negatively impacted patient outcomes (9).
A model, from 2017, estimated that rejected specimens result in $357.15 per hospital inpatient and $337.05 per outpatient annually, accounting for approximately 0.23% to 1.2% of total hospital operating expenses (10). A comprehensive approach to preanalytical quality ensures reliable results and minimizes the risk of delays or misdiagnoses.
Don’t settle for solving part of the problem - aim for total preanalytical excellence.
References:
- Authority, P.P.S. (no date) In vitro hemolysis: Delays may pose safety issues advisory. https://patientsafety.pa.gov
- Buño, A. and Oliver, P. (2021) 'POCT errors can lead to false potassium results,' Advances in Laboratory Medicine / Avances En Medicina De Laboratorio, 3(2), pp. 142–146. https://doi.org.
- Lin Y et al. Preanlytical Phase errors constitute the vast majority of errors in Clinical Laboratory Testing. Clinical Chemistry, Volume 70, Issue Supplement_1, October 2024, hvae106.207, https://doi.org
- Ana-Maria Simundic AM et al. The prevalence of preanalytical errors in a Croatian ISO 15189 accredited laboratory. In Clinical chemistry and laboratory medicine 48 (7), pp. 1009–1014. DOI: 10.1515/CCLM.2010.221.
- Clinical and Laboratory Standards Institute (CLSI) (February 2009): Blood gas and pH Analysis and Related Measurements; Approved Guideline - second edition. CLSI document C46-A2 (ISBN 1-56238-694-8).
- Lippi, Giuseppe; Ippolito, Luigi; Fontana, Rossana (2011): Prevalence of hemolytic specimens referred for arterial blood gas analysis. In Clinical chemistry and laboratory medicine 49 (5), pp. 931–932. DOI: 10.1515/CCLM.2011.136.
- Getawa, Solomon; Aynalem, Melak; Melku, Mulugeta; Adane, Tiruneh (2023): Blood specimen rejection rate in clinical laboratory. A systematic review and meta-analysis. In Practical laboratory medicine 33, e00303. DOI: 10.1016/j.plabm.2022.e00303.
- Casati, M. et al. (2022) 'Hemolysis and blood gas analysis: it’s time for a change!,' Scandinavian Journal of Clinical and Laboratory Investigation, 82(2), pp. 138–142. https://doi.org.
- Lee, N.Y. (2019) 'Reduction of pre-analytical errors in the clinical laboratory at the University Hospital of Korea through quality improvement activities,' Clinical Biochemistry, 70, pp. 24–29. https://doi.org.
- Rooper, L. et al. (2016) 'Targeting rejection: Analysis of specimen acceptability and rejection, and framework for identifying interventions in a single tertiary healthcare facility,' Journal of Clinical Laboratory Analysis, 31(3). https://doi.org.
Cookies are used on this website
Use of cookiesPlease enter a valid email
We will be sending an e-mail invitation to you shortly to sign in using Microsoft Azure AD.
It seems that your e-mail is not registered with us
Please click "Get started" in the e-mail to complete the registration process
Radiometer is using Microsoft AZURE Active Directory to authenticate users
Radiometer uses Azure AD to provide our customers and partners secure access to documents, resources, and other services on our customer portal.
If your organization is already using Azure AD you can use the same credentials to access Radiometer's customer portal.
Key benefits
- Allow the use of existing Active Directory credentials
- Single-sign on experience
- Use same credentials to access future services
Request access
You will receive an invitation to access our services via e-mail when your request has been approved.
When you accept the invitation, and your organization is already using AZURE AD, you can use the same credentials to access Radiometer's customer portal. Otherwise, a one-time password will be sent via e-mail to sign in.
